Bohr Model Vs Electron Cloud Model
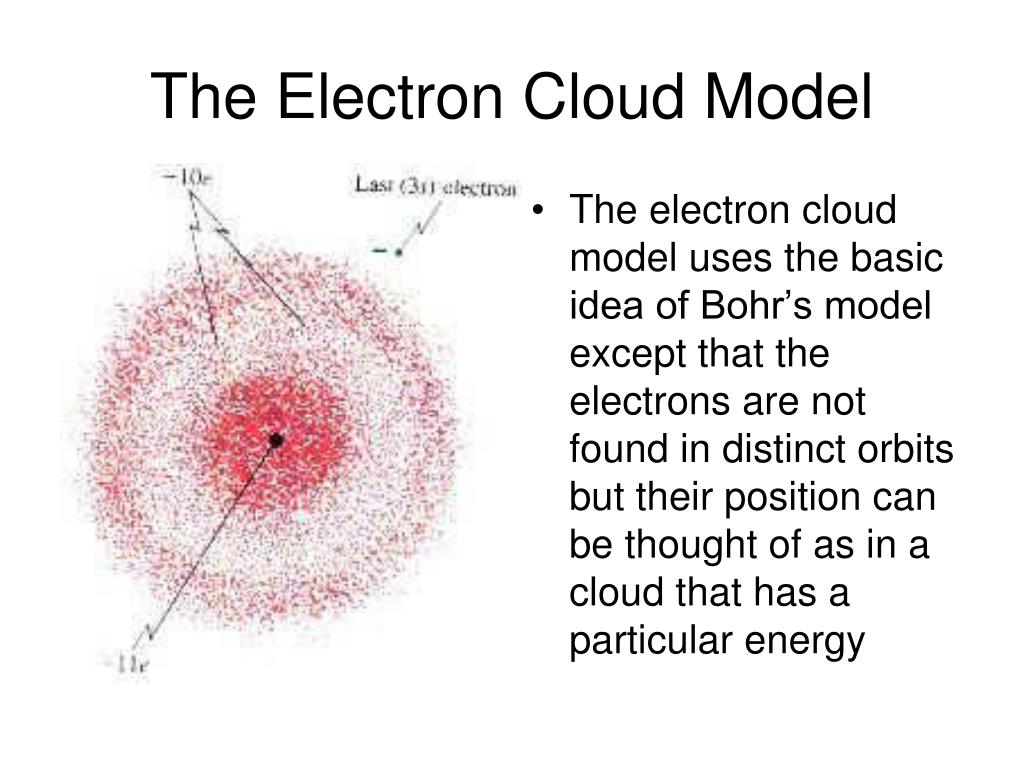
Bohr model vs electron cloud model. In the other way the cloud model treats the energy levels as probability of electron clouds in which electrons are to be expected to exist in that area or regions. The main difference between Rutherford and Bohr model is that Rutherford model does not explain the energy levels in an atom whereas Bohr model explains the energy levels in an atom. Get an answer for What is the similarities and differences between the current electron cloud model and the Bohr model of the atom and find homework help for.
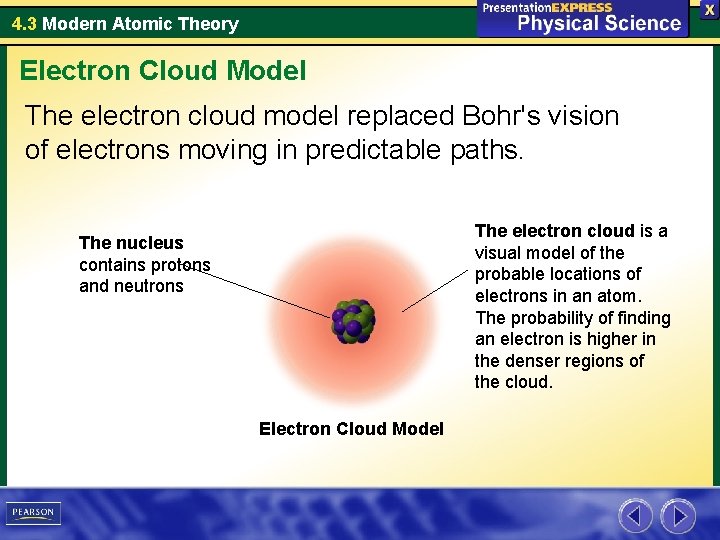
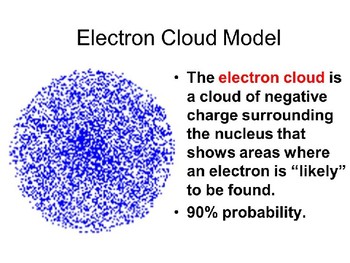
The Electron Cloud Model was of the greatest contributions of the 20th century leading to a revolution in physics and quantum theory. Ap1 Bohr model hydrogen. In the Schrödinger picture the operators stay fixed while the Schrödinger equ.
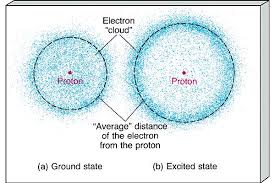
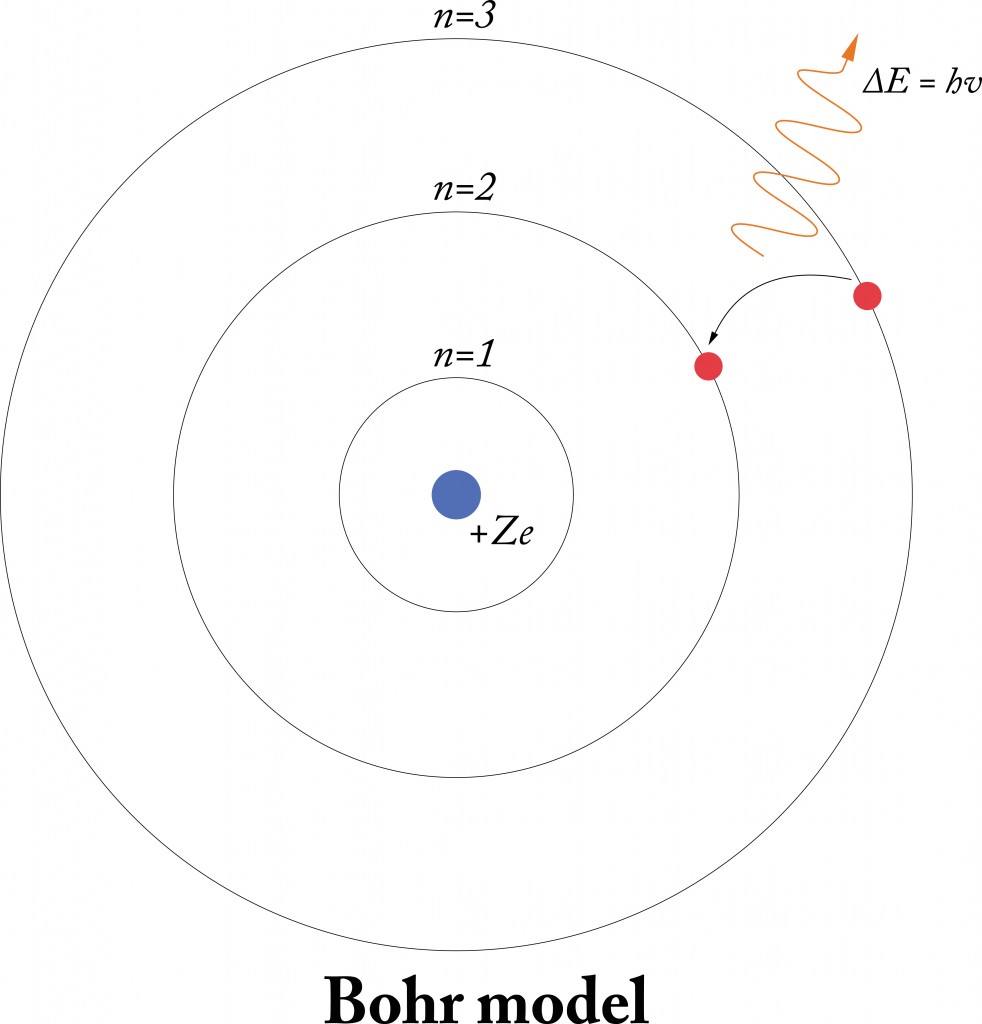
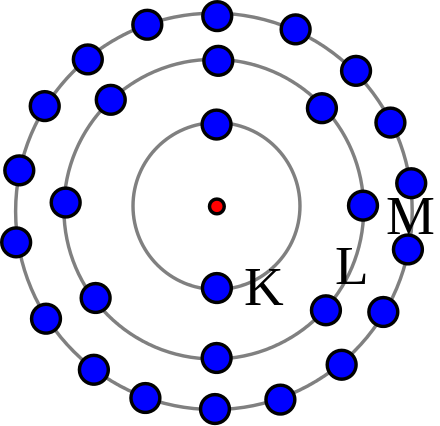
In the Bohr model an electrons position is known precisely because it orbits the nucleus in a fixed path. Because vacuum electromagnetic energy around a single electron is NOT energy. First as shown on this page Bohrs single electron does NOT fall into nucleus just by acceleration.
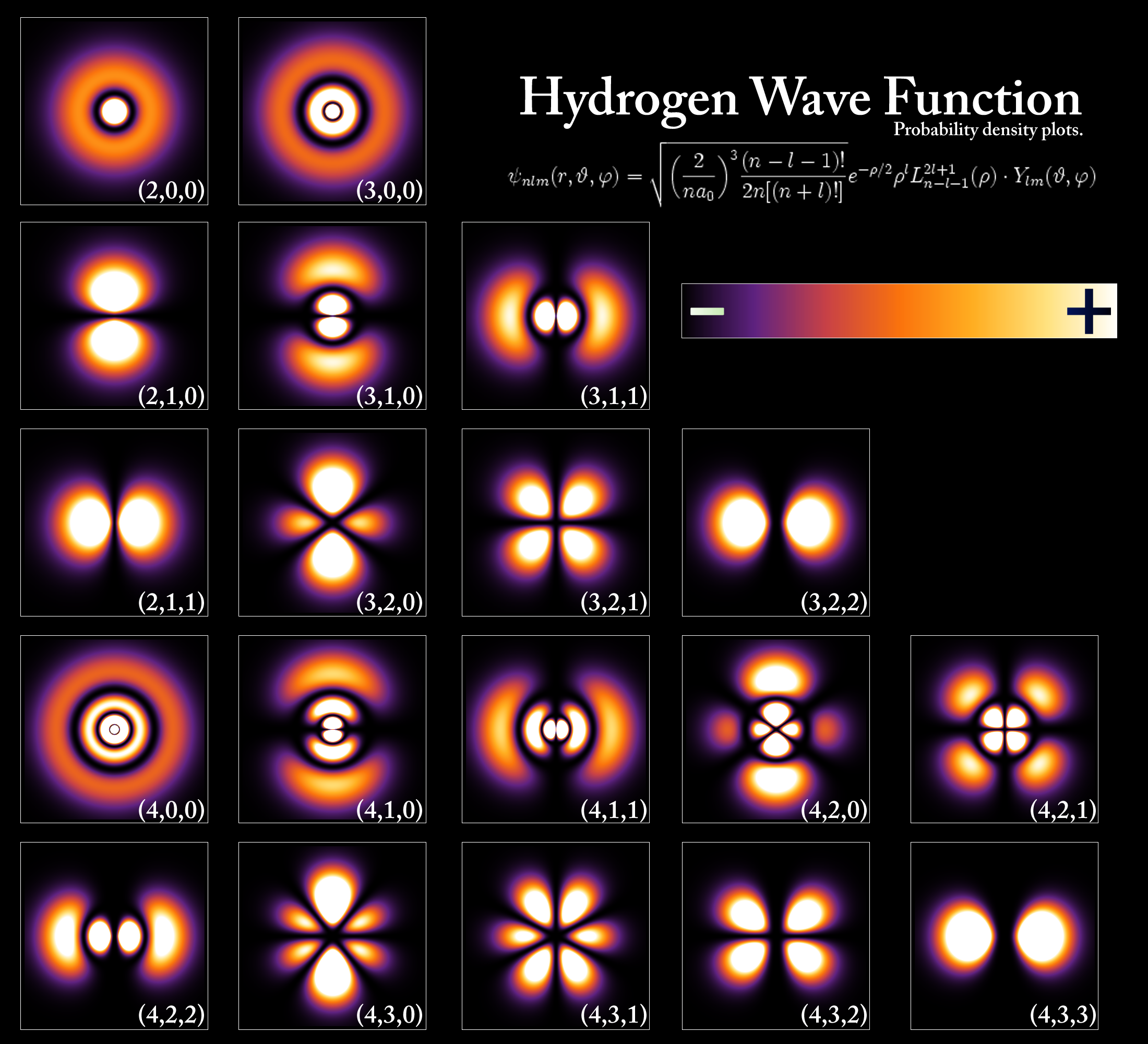
In my understanding the Bohr model also proposes electron orbits with fixed radii. The electron cloud model uses the concept of orbitals referring to regions in the extra-nuclear space of an atom where electrons are likely to be found. The key difference is that in most modern interpretations of the Schrodinger model the electron of a one-electron atom rather than traveling in fixed orbits about the nucleus has a probablity distribution permitting the electron to be at almost all locations in space some being much more.
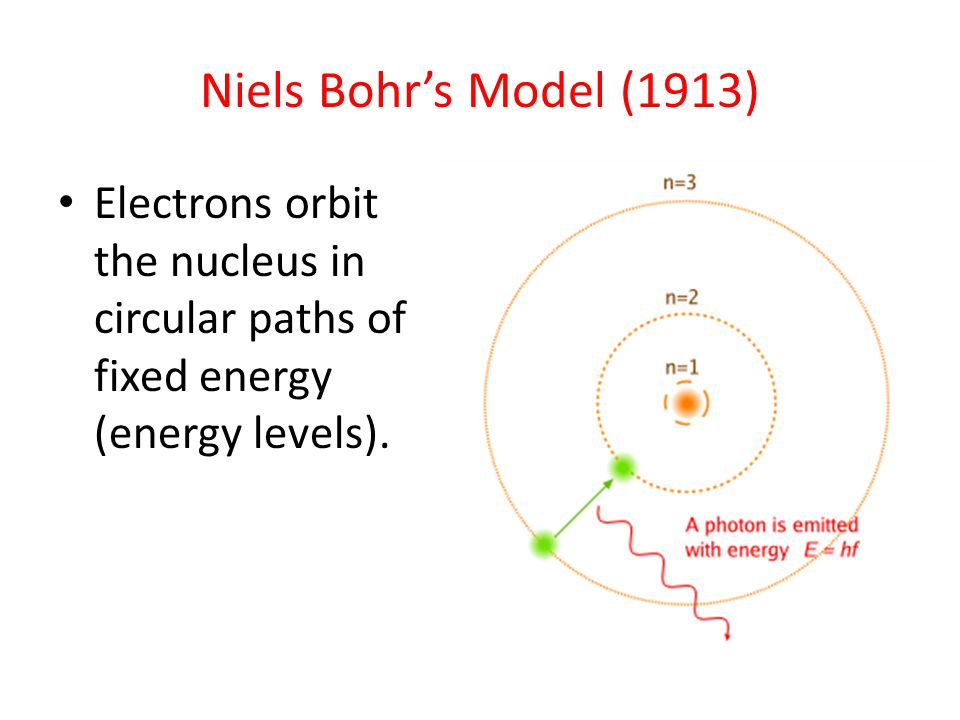
Bohrs model treats electron energy level as evidently well-defined as an orbital path surround the nucleus similar to a model just like the way planet is encircling the Sun. Bohr model was proposed by Niels Bohr in 1915. Observables are represented by Hermitian operators which act on the wave function.
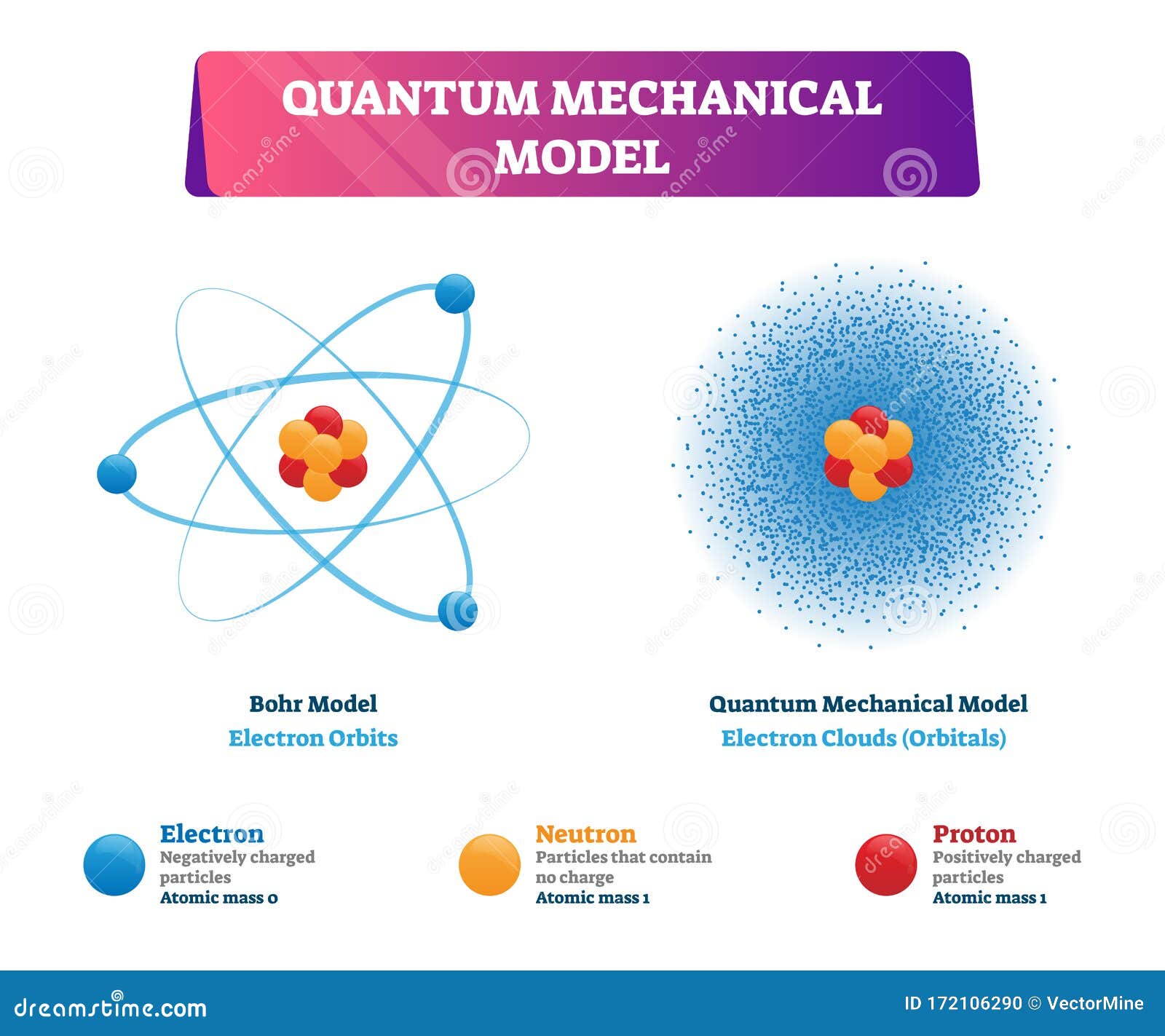
There have been many models constructed by scientists who have tried to figured out how an atom is made up and what exactly it is made up of but two of the best atomic models of the atom are Bohrs atomic model and the Electron cloud model which were both made in the early 1900s. Bohr model is considered as a modification of Rutherford model. In order for an electron to be in the electron cloud of an atom it must be in one of the allowable orbitals and.
An orbital is a mathematical function that describes the wave-like behavior of electrons in an atom. In the Quantum Mechanical Model the electron is treated mathematically as a wave.
Bohrs model is more like a halfway point between the electron cloud model and the incomplete but commonly taught solar system model where the electrons literally rotate around a.
The energy level solutions are the same for both. In the Quantum Mechanical Model the electron is treated mathematically as a wave. In the Bohr Model the electron is treated as a particle in fixed orbits around the nucleus. Bohr model was proposed by Niels Bohr in 1915. In Schrodingers Theory- Quantum systems are regarded as wave functions which solve the Schrödinger equation. In the other way the cloud model treats the energy levels as probability of electron clouds in which electrons are to be expected to exist in that area or regions. Bohrs model is more like a halfway point between the electron cloud model and the incomplete but commonly taught solar system model where the electrons literally rotate around a. Observables are represented by Hermitian operators which act on the wave function. Note that this model still has one proton and one electron in a.
Bohrs model treats electron energy level as evidently well-defined as an orbital path surround the nucleus similar to a model just like the way planet is encircling the Sun. In my understanding the Bohr model also proposes electron orbits with fixed radii. In the Bohr model an electrons position is known precisely because it orbits the nucleus in a fixed path. The potential energy function is the same for both. Get an answer for What is the similarities and differences between the current electron cloud model and the Bohr model of the atom and find homework help for. Bohrs model treats electron energy levels as clearly defined orbital paths around the nucleus ike planets orbit the Sun. Observables are represented by Hermitian operators which act on the wave function.




























Post a Comment for "Bohr Model Vs Electron Cloud Model"